NEOSAFE
There remains a critical gap in the design and availability of medical devices suitable for the neonatal population, particularly for procedures such as ventilation, enteral access, and dialysis. Existing equipment is poorly suited for infants due to limited safety validation and improper scaling, often leading to clinical compromises. High development costs and a small market discourage large manufacturers from addressing this unmet need. This project explored the use of 3D printing as a practical and cost-effective approach to rapidly manufacture patient-specific neonatal medical devices. The flexibility and precision of additive manufacturing offer a promising pathway to improve outcomes for this vulnerable population through on-demand, anatomically scaled instrumentation.
Novel Material for Manufacturing of Neonatal Devices via 3D Printing
Year: 2016
Customer: NIH
The Puzzle
What was the challenge? Why was it worth solving?
Despite rapid advancements in neonatal care, the most fundamental challenge persists: the absence of properly scaled, biocompatible, and safe medical devices tailored for premature and newborn infants. Current commercial options often fail in critical ways:
Improper Fit & Functionality: Standard face masks frequently leak or obstruct the airway, compromising ventilation and often going unnoticed during care.
Dialysis Limitations: Neonatal dialysis catheters are too large, too rigid, or entirely unavailable, limiting renal therapy options for the smallest patients.
Material Challenges: Existing 3D-printable polymers (e.g., acrylics) are cytotoxic, and most printable materials are far too stiff for neonatal use, lacking both flexibility and safety.
Manufacturing Gap: No known polymer-process combination currently exists to reliably fabricate neonatal devices like tracheostomy tubes or catheters using additive manufacturing.
The core puzzle was to identify and tailor a material-process pairing that meets all the demands of neonatal device fabrication: biocompatibility, sterilizability, appropriate mechanical behavior, and scalability, within a low-volume, flexible manufacturing platform.
The Game Plan
How the problem was approached — strategy, tools, and intent.
The effort focused on developing a UV-curable, medical-grade silicone formulation suitable for vat photopolymerization to fabricate neonatal-specific medical devices. This work addressed the critical lack of flexible, biocompatible materials that can be reliably processed using additive manufacturing for premature and newborn populations.
The project plan included:
Formulating a photocurable silicone resin by combining vinyl-terminated silicones with appropriate solvents and photoinitiators.
Optimizing resin properties for vat photopolymerization.
Demonstrating 3D printing and UV-curing of flexible catheter tubing as a proof-of-concept neonatal device.
Evaluating printed samples for mechanical performance, sterilizability, and biocompatibility.
This foundational work will enable small-volume, patient-specific production of critical neonatal devices and lay the groundwork for future expansion into additional applications.
What Actually Happened
The results. The breakthroughs. Maybe even a few surprises.
Over the course of the project, nearly 600 formulations were synthesized and evaluated. Of those, 192 demonstrated successful UV curing. It became clear that formulation composition, including the type of silicone, photoinitiator, and solvent, had a direct impact on mechanical properties, cure behavior, and biocompatibility.
Mechanical performance was improved through formulation optimization, including the addition of reinforcement agents, oxygen scavengers, and tuning of curing parameters. Importantly, specimens retained structural integrity post steam sterilization, and the final cured materials received a biocompatibility grade of “0” per ISO 10993 standards.
Multilayer fabrication was successfully demonstrated with strong interlayer adhesion, and the formulations were processed using vat photopolymerization, confirming printability. These results validated the approach and highlight the potential of these materials for further development toward neonatal medical device manufacturing.
Show & Tell
Visuals, prints, textures, data — what it looked like in the real world.



Not every experiment ends with applause, some cured into mystery blobs. A good reminder that formulation work is equal parts science and humility.
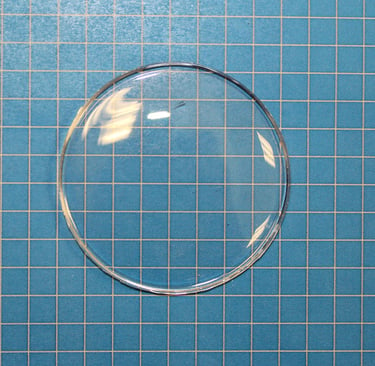
Clear, cylindrical, multilayered, and cured - this specimen is exactly what we aimed for. Proof that the process works when the formulation is just right.
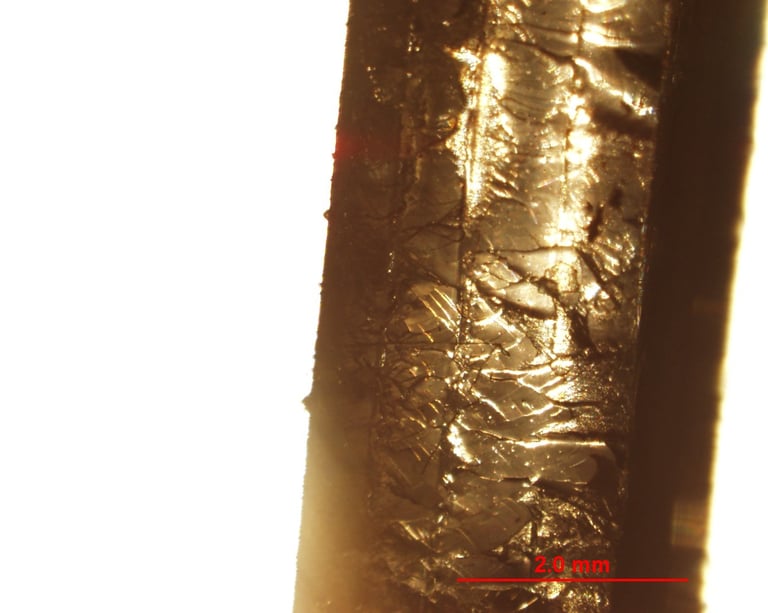
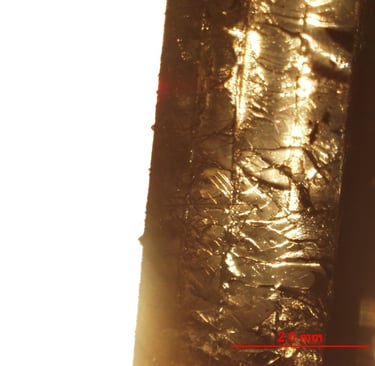
A closer look at the multilayer specimen, because who doesn't love a well-organized polymer stack under the microscope?
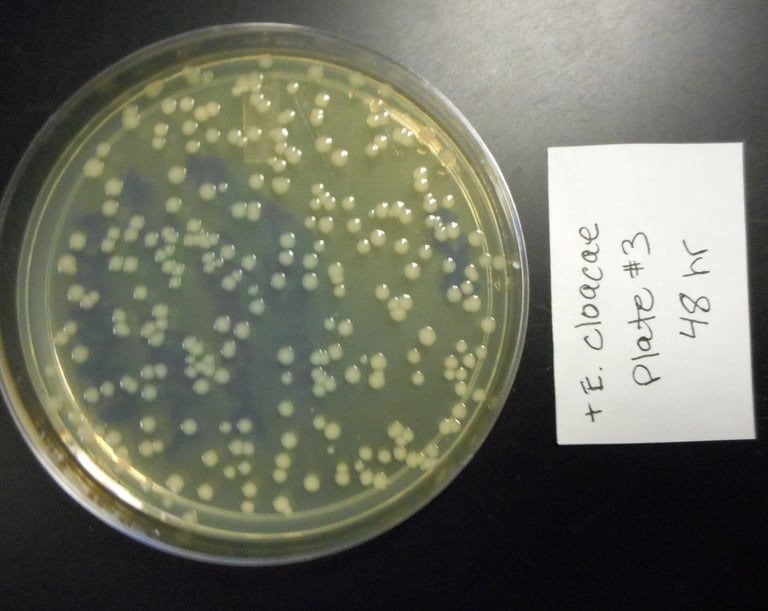
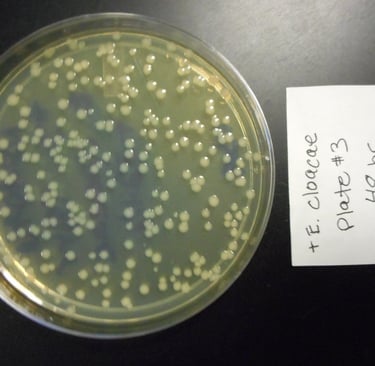
Petri dish showdown: 80 hours later, the bacteria didn’t stand a chance. Early signs of potential antibacterial performance worth exploring.
Lessons from the Lab
What worked. What didn’t. What was learned along the way.
Formulation is a balancing act. Developing a UV-curable silicone that is printable, biocompatible, and sterilizable required iterative tuning of solvent ratios, photoinitiators, and polymer backbones. Even small adjustments had significant effects on curing behavior and mechanical integrity.
Cure ≠ Success. While hundreds of formulations cured under UV light, only a subset met the mechanical and biocompatibility benchmarks required for neonatal use. Just because something solidifies doesn’t mean it’s ready for a catheter.
Mechanical strength is tunable. Post-curing conditions, additive inclusion, and oxygen control played a measurable role in final material performance. Reinforcement isn’t just about filler - curing dynamics matter.
Biocompatibility is non-negotiable. The best formulations earned ISO 10993 Grade “0” - no irritation, no cytotoxicity. That bar is high, but it’s essential for neonatal applications.
Printing and performance can co-exist. The successful printing of multilayered specimens with clean layer adhesion demonstrated that material formulation and vat photopolymerization can work hand-in-hand, when both are carefully engineered.